The mission of the Society is to improve patient care and outcomes by facilitating implementation of functional assays into clinical care. The purposes are to foster research and development of functional precision medicine solutions across medicine; to accelerate the dissemination of new and relevant research findings among interested parties; to promote education and training about functional precision medicine; to foster solutions for clinical testing of functional precision medicine approaches; and to improve efficiency of adoption of functional precision medicine solutions through interaction with academia, regulatory bodies, industry, and patients.
Become a Member

PMNET Forum 2024 - October 10-11, Riga Latvia
Join SFPM members at the next PMNET Forum! Participation is complimentary. This forum is an in-person event and has a limited availability. Registration is compulsory and is made on a first come-first served basis.
Registration includes:
- Admission to the program
- Coffee breaks on October 10-11
- Lunch on October 10-11
March 11 - 13, 2025
Hyatt Regency Boston
Boston, Massachusetts
CONFERENCE CO-CHAIRS:
Anthony G. Letai, Dana-Farber Cancer Institute, Boston, Massachusetts
Elaine R. Mardis, The Institute for Genomic Medicine at Nationwide Children’s Hospital, Columbus, Ohio
Peter Horak, German Cancer Research Center, Heidelberg, Germany
Alice Soragni, University of California, Los Angeles, California
FUNCTIONAL AND GENOMIC PRECISION MEDICINE IN CANCER: DIFFERENT PERSPECTIVES, COMMON GOALS
Precision medicine applied to oncology aims to match each patient to the right therapy. Heretofore, genomics has been the main tool in performing precision medicine. Yet, many cancer patients lack actionable alterations as there is a limited set of highly predictive biomarkers to accurately match patients to effective therapies. There is a need to extend the benefits of precision medicine to a larger proportion of cancer patients. Therefore, additional strategies need to be explored. One important approach is functional precision medicine, a strategy by which living patient tumor cells are exposed to therapies and signals measured to predict clinical response. The goal of this AACR Special Conference is to share experiences among those in the genomics and functional precision oncology arenas, to accelerate the progress toward routine clinical adoption of combined approaches in the oncology clinic. At this exciting program in Boston we will share technical, clinical, and logistical experiences, as well as our understanding of the barriers to widespread adoption of genomic and functional precision medicine in the treatment of the cancer patient, and how these approaches might be combined to provide more powerful tools than either alone.
Do you want to help shape the future of the SFPM? Self-nominate or nominate a peer for a board position today and lend your experience and input!
Open positions for this election cycle include
Positions will be filled by vote of the Professional or Associate members in good standing. You must be a Professional or Associate member to view the form and apply.
The SFPM arose out of recognition of the unmet need in matching cancer patients to the therapies best suited for them. Through the use of functional assays, we hope to bridge the gap between patients and the treatments best suited for their cancer. This is possible through research, communication, and collaboration amongst our members and with outreach to the cancer therapy community as a whole. To further the mission, we offer our members the following exclusive benefits
Applications for membership may be submitted at any time during the year. We look forward to having you onboard!
Come join us for our Virtual Monthly Seminar which will bring together a unique mix of pharmaceutical, academic and technology leaders, the series should not be missed for those interested in the use of assaying technology to further biomarker discovery, drug R&D and personalized healthcare outcomes.
Wednesday, March 11, 2026
12:00 PM EST
Meeting URL: www.zoom.us
Meeting ID: 828 5622 5783
Passcode: 027718

Stay connected, stay ahead. Know everything that matters, as it happens. From the very latest industry news, reports, papers, and studies. Would you like to see your news here? Email the admin and we will get it linked. Check out our news archive for all past stories and posts.

The Stuart and Molly Sloan Precision Oncology Institute at Fred Hutch Cancer Center is excited to announce our next event: Functional Precision Oncology Symposium, co-hosted by Dr. Chris Kemp, Professor, Human Biology Division, Fred Hutch Cancer Center and Dr. Venu Pillarisetty, Professor, Surgical Oncology, University of Washington School of Medicine. This symposium will highlight current efforts on the application of ex vivo functional testing for both translational research and clinical applications, with sessions exploring technical and computational advances, integration of functional testing with genomics, clinical and structural roadblocks to implementing functional testing, and clinical trial results from both liquid and solid tumors.

Scientific Workshops are interactive discussions covering the latest scientific developments in a range of hematologic topics. The 2025 Scientific Workshops will take place on Friday, December 5, and will also be streamed on the ASH annual meeting platform for virtual participants.

Anthony Letai, MD, Ph.D., was sworn in today as director of the National Cancer Institute (NCI), part of the National Institutes of Health (NIH), by Health and Human Services Secretary Robert F. Kennedy, Jr.
Dr. Letai takes the helm of the world’s most prestigious cancer research agency after serving as professor of medicine at Harvard Medical School and medical oncologist at the Dana-Farber Cancer Institute. He possesses decades of experience studying cell death in cancer, developing treatments, and identifying predictive biomarkers.

In this riveting talk, SFPM Board Member Dr. Diana Azzam, a cancer researcher and the Scientific Director of the Center for Advancing Personalized Cancer Treatments at Florida International University, shows how functional precision medicine and AI can be used to develop individualized treatmentplans for patients who have exhausted standard cancer care. By testing each patient’sown cancer cells to find treatments that work best for them, her work bridges research and clinical care, bringing hope to cancer patients through personalized medicine. Diana Azzam, PhD is a cancer researcher and Scientific Director of the Center for Advancing Personalized Cancer Treatments at Florida International University. Her lab uses functional precision medicine and AI to develop individualized treatment plans for patients who have exhausted standard care--testing each patient’s own cancer cells to find treatments that work best for them. Her work bridges research and clinical care, bringing hope to cancer patients through personalized medicine. This talk was given at a TEDx event using the TED conference format but independently organized by a local community.
In recent announcements, the United States National Institutes of Health announced that it wants to prioritize human-based research technologies. In a key adjunct to this initiative, it stated that it would no longer develop funding opportunities focused solely on animal models of human disease. The NIH release specifically mentions the use of “organoids, tissue chips and other in vitro systems that allow scientists to model human disease and capture human variability and patient-specific characteristics.” Members of the Society for Functional Precision Medicine should recognize that this announcement will likely increase the interest in their work, as nearly all members are developing human based models. The SfPM suggest that members stay alert for NIH funding opportunities that may later accompany this new initiative, as well as for signs that other countries are moving in the same direction. Australia is an example of a country whose national science agency (CSIRO) has previously reported favorably on the use of non-animal models.
The terms “non-animal models” and “human models” are starting to be used interchangeably. Examples of human models in which members of the Society of Functional Precision Medicine have expertise include organoids, 2D cultures, spheroids, organ on chip or tumor on chip, implantable microdevices, single cell suspensions, and tissue slices.

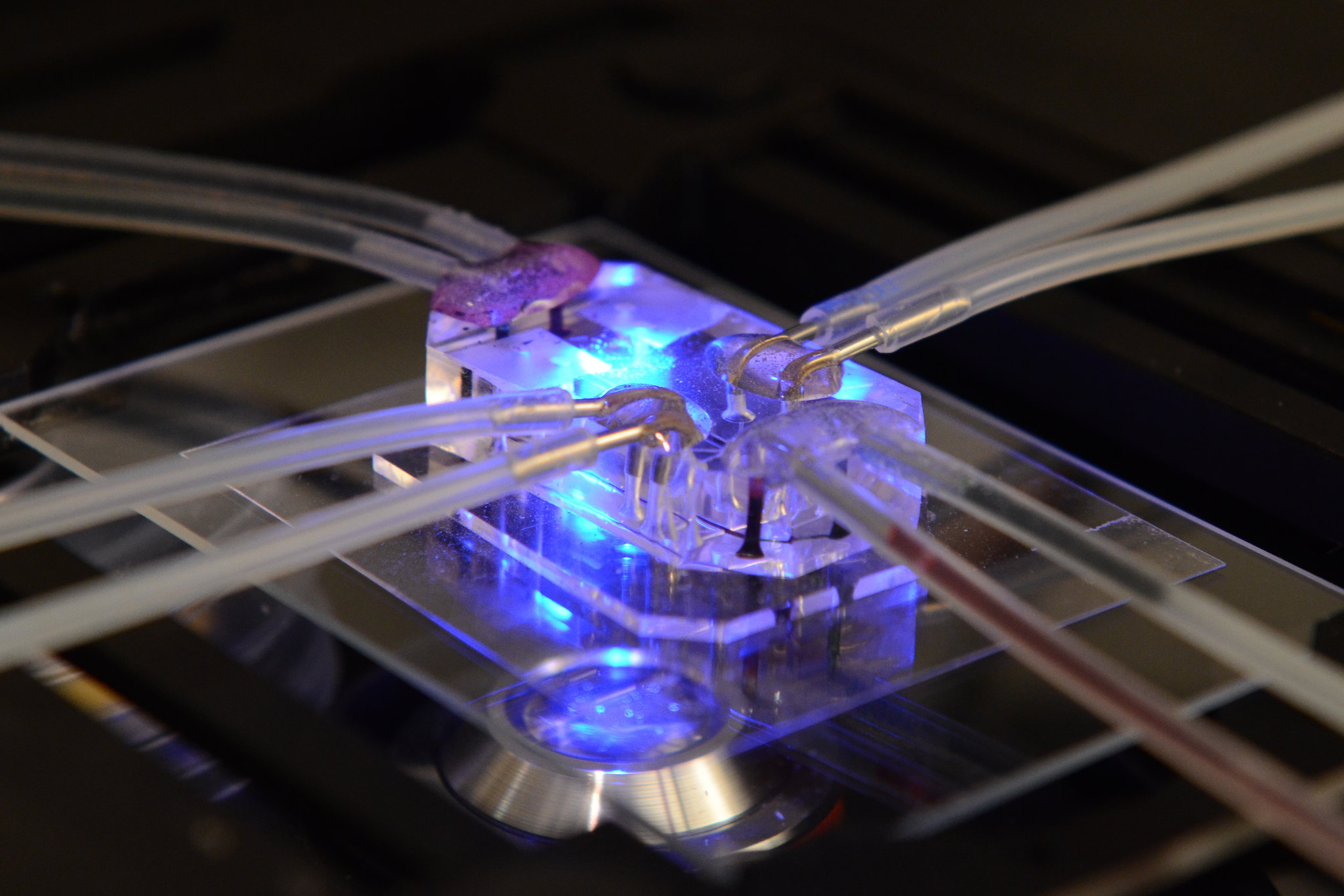
Cell density, the ratio of cell mass to volume, is an indicator of molecular crowding and a fundamental determinant of cell state and function. However, existing density measurements lack the precision or throughput to quantify subtle differences in cell states, particularly in primary samples. Here we present an approach for measuring the density of 30,000 single cells per hour by integrating fluorescence exclusion microscopy with a suspended microchannel resonator.
The SFPM is growing, and we need financial support. While we collect annual individual membership dues, these only fund a fraction of our needs. To plan for the future, the SFPM must secure a stable financial basis from which to plan. Even though the Board is made up of unpaid volunteers, we nonetheless have significant costs to defray, particularly with respect to annual meetings and administrative support.
Contributions from all tiers will be recognized by name on the SFPM website, however, contributions at the Precision tier will receive preferred logo placement and recognition on the SFPM website, logo and name recognition at each monthly seminar, and institution name and logo prominently displayed at our annual meeting and on meeting marketing materials. Precision tier supporters will also receive ten (10) complimentary memberships to be distributed amongst their institutions. Functional tier supporters will receive name and logo recognition during at least six of our monthly seminars as well as name and logo recognition on our website. Functional tier supporters will also receive five (5) complimentary memberships to be distributed amongst their institutions. Advocate tier supporters will receive name and logo recognition on our website and one (1) complimentary membership. We hope you agree with us that “the future is functional”, and that the SFPM is getting us there faster.






